Cannot Access Article to Review on Gait & Posture
Introduction
The incidence of spinal cord injury (SCI) worldwide is between 250,000 and 500,000 individuals each year (Quadri et al., 2020). In Western European countries traumatic SCI incidence is of 16 to 19.4 new cases per million inhabitants per year (Scivoletto et al., 2017). Walking is usually affected in patients with SCI co-ordinate to the lesion level and the resulting unlike levels of muscle paralysis, sensory impairment, spasticity, and the lack of trunk command (Bani et al., 2013). In the field of SCI research, there is an emphasis on the ability to ambulate equally a functional outcome and every bit an indicator of quality of life (Jackson et al., 2008), particularly in individuals with incomplete SCI (iSCI) (Ditunno et al., 2008).
Walking role recovery is tackled through several therapeutic interventions such as surgery, physiotherapy, medications, orthotics, and robotics in which precise evaluation of walking function is mandatory (Scivoletto et al., 2011). Periodic gait measurements can exist used to evaluate the response to these therapeutical approaches (McGinley et al., 2009), to assess changes in walking over time, and to discriminate between normal and altered gait (Baker, 2006). In this regard, three-dimensional (3D) kinematic gait assay tin can provide useful data to guide rehabilitation interventions to improve walking function of people with traumatic and non-traumatic iSCI (Irish potato et al., 2019). Nevertheless, isolated kinematic parameters do not provide a full picture of gait pattern impairment (Guzik and Drużbicki, 2020), and on the other mitt, it may be difficult to describe considerately the heterogeneity of the different gait abnormalities present in iSCI and to quantify the degree by which they deviate from normal gait patterns. The Gait Difference Alphabetize (GDI) is a multivariate measure of overall gait pathology based on xv gait features built upon 3D kinematic data originally designed from a sample of children with cerebral palsy (CP) (Schwartz and Rozumalski, 2008). The GDI is a dimensionless parameter represented equally a unmarried score for an individual gait divergence from a normative reference group, which aims at providing a comprehensive, easy to interpret, and clinically meaningful metric of overall gait function.
The usefulness of GDI has been assessed through correlations with clinically-validated gait scales. Concurrent and face up validity of GDI was firstly carried out by comparison with the Gillette Functional Assessment Questionnaire walking scale (FAQ) and topographic classifications of CP in children population (Schwartz and Rozumalski, 2008). Afterward, the relationship between the GDI, Gross Motor Function Measure out (GMFM), and Gross Motor Role Classification System (GMFCS) in a representative sample of ambulatory children with CP provided greater validity to the GDI (Molloy et al., 2010). The ability of the GDI to distinguish between GMFCS levels in children with CP in the study adult by (Massaad et al., 2014) concurred with those plant by Molloy et al. (2010) and by Schwartz and Rozumalski (2008) for the FAQ. Furthermore, confront validity of the GDI in adults with CP was demonstrated past comparing with GMFCS (Maanum et al., 2012), which showed similar distributional properties as those reported in children with CP. The GDI was able to distinguish different levels of gait impairment in adults (Maanum et al., 2012) and children (Schwartz and Rozumalski, 2008; Molloy et al., 2010; Massaad et al., 2014) with CP. However, no correlations have been published between the GDI and other valid walking ability issue measures commonly used in clinical settings to appraise gait variability in adult population with SCI.
The Walking Index for Spinal Cord Injury (WISCI) II is a walking scale specifically developed for iSCI population composed of 21 levels (Dittuno et al., 2001), in which levels are ordered by degree of an individual's walking impairment, from most impaired to least impaired (Ditunno et al., 2007), integrating a hierarchical guild for the use of ambulatory assistive devices (AADs), orthoses, and the physical assist needed to complete a 10 m walking distance. WISCI II levels differs from cocky-selected (SS) WISCI, defined as patient'due south preferential condition to walk in the customs or the household, and maximum WISCI, which is related to the highest level at which a person can safely walk 10 m (Burns et al., 2011). The WISCI 2 is a valid (Morganti et al., 2005; Ditunno et al., 2007), reliable (Marino et al., 2010; Scivoletto et al., 2014), and responsive (van Hedel et al., 2006) outcome measure to appraise walking ability in people with SCI. In our best knowledge, there is no scientific literature which have studied the relationship between the GDI and the WISCI Ii in developed population with SCI.
The GDI has been used as an result measure to study gait in several weather condition such as: CP (Schwartz and Rozumalski, 2008; Molloy et al., 2010; Cimolin et al., 2011; Sagawa et al., 2013; Massaad et al., 2014; Wilson et al., 2015; Malt et al., 2016; Ito et al., 2019; Rasmussen et al., 2019), post-stroke hemiparetic gait (Correa et al., 2017; Guzik and Drużbicki, 2020), Duchenne muscular dystrophy (Sienko Thomas et al., 2010), Parkinson's disease (Galli et al., 2012; Speciali et al., 2013), arthritis (Broström et al., 2013; Esbjörnsson et al., 2014; Rosenlund et al., 2016; Kobsar et al., 2019; Bazarnik-Mucha et al., 2020), lower limb amputations (Eshraghi et al., 2014; Kark et al., 2016), degenerative spinal pathologies (Mar et al., 2019; Trivedi et al., 2021; Zhou et al., 2021), diverse genetic (Ito et al., 2020; Mindler et al., 2020) and built disorders (Eriksson et al., 2015; Garman et al., 2019), and even the effect of the COVID-19 on physical function (Ito et al., 2021), amid others. A recently published article past Hwang et al. (2021) used the GDI equally a way to quantify and characterize gait patterns in ambulatory children and adolescents with transverse myelitis, whose gait showed moderate kinematic deviations from normal gait blueprint. Even so, to date, no work has been published regarding the validity of the GDI in population with SCI.
Joint kinematics and spatiotemporal gait parameters differ between adult and child population due to the maturation and aging processes of the gait, associated to the neuromuscular evolution and the changes in strength that occur during adolescence and machismo (Cupp et al., 1999; Ganley and Powers, 2005). In this regard, information technology is necessary to consider the functional differences of the gait pattern in relation to the mature stage in people with SCI.
The aim of the present report was to evaluate the relationship betwixt the GDI and WISCI 2 levels in developed population with iSCI. Our hypothesis was that the most altered gait kinematics of people with iSCI, reflected by GDI values below 100, would be associated with lower scores of the WISCI 2.
Materials and Methods
Study Design
An observational retrospective report was conducted on a database of 3D kinematic gait assay of adult population composed by patients with iSCI and healthy volunteers (HV) gathered between Baronial 2019 and July 2021 at the Biomechanics and Technical Aids Unit of the National Infirmary for Paraplegics of Toledo (Spain). All the individuals recruited for the study signed informed consent to participate in the written report. Co-ordinate to the Announcement of Helsinki, all participants were informed about the purpose and form of the study, and about their rights to withdraw from the written report. The study protocol was reviewed and canonical by the Local Ethics Commission of Academy Hospital Complex of Toledo, Spain.
Participants
Patients included in the report met the following inclusion criteria: (i) subjects anile 16 years or over; (ii) having suffered a SCI regardless of the etiology (traumatic or non-traumatic), time since injury onset, and neurological level of injury (NLI); (3) classified as C, D, or East by the American Spinal Injury Association (ASIA) Harm Scale (AIS) (Kirshblum et al., 2011); (iv) with the power of walking x g independently with any type of external assistance required (orthoses, crutches or canes); (v) with SS WISCI 2 levels nerveless; and (half-dozen) capacity to be informed and give consent to participate in the study. Patients from the database were excluded of the study if they followed one of the unlike weather condition: (i) having suffered from rheumatic, orthopedic, or other neurological disorders exterior of SCI that affected gait; (2) need for support in parallel bars, walker and/or physical assistance required of ane or two people to walk 10 1000 safely; (three) psychiatric or cerebral atmospheric condition that may have interfered with the performance of the gait analysis.
Based on the medical history reported past HV in the recruitment process, they were excluded if they experienced musculoskeletal or neurological disorders that affected gait. 3D kinematic information acquired from HV were used to calculate an boilerplate normal value of gait kinematics and hence to calculate the deviation from normal gait pattern for each patient, in essence, the GDI.
Experimental Protocol
3D kinematic gait data were obtained with Codamotion motility capture system (Charnwood Dynamics, Ltd., United Kingdom), comprised of 22 active markers placed on the lower limbs (Figure 1), 3 scanners, and 2 Kistler force platforms embedded in a ten-m walkway. Markers were positioned on the following anatomical references: sacrum (two lateral markers), inductive superior iliac spines (ASIS), posterior superior iliac spines (PSIS), lateral surface of the thighs (anterior and posterior femur markers), lateral femoral condyles, lateral surface of the legs (anterior and posterior tibia markers), lateral malleoli, calcaneus (posterior lateral heels), and fifth metatarsal heads. Marking trajectories were collected at a sampling frequency of 200 Hz. A 3D skeletal model was created for each individual based on markers placement and anthropometric measures taken for each subject area, which included: weight, height, pelvis width and depth, knees and ankles width. Subjects were informed to walk naturally at their SS speed with the minimum external help required -canes, crutches, and/or orthoses-. A valid step was considered as the one in which each foot was on a dissimilar forcefulness platform. 5 complete gait cycles or three complete cycles in those individuals with SCI who were not able to get 5 valid cycles were collected, time-normalized and averaged. A total of 302 and 446 strides were collected for the grouping with iSCI and the HV group, respectively. The consummate records were then processed using the software for information analysis ODIN v.two.02 (Codamotion Ltd., Great britain) to calculate the mean values of 3D kinematic parameters for the gait cycle of the right and left leg, for pelvis, and hip, articulatio genus, and ankle joints.
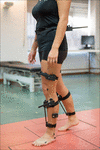
Figure one. Placement of the 22 active markers of Codamotion motility capture system on the lower limbs of an individual from HV group.
Data Analysis
The GDI is calculated upon the procedure described in Schwartz and Rozumalski (2008). The index is derived from a set of 9 kinematic curves of a unmarried stride: i) pelvic orientation and hip angles in the 3 planes of space (sagittal, frontal and transversal), (ii) knee flexion and extension, (iii) ankle dorsiflexion and plantar flexion, and (iv) foot progression angle.
In the original study (Schwartz and Rozumalski, 2008), a dataset with more than 6,000 strides of patients with CP was used to calculate a 15-feature footing to account for 98% of the total variation of the whole dataset and to allow to reconstruct the kinematic gait curves with a 98% allegiance on boilerplate. This footing immune to calculate the representation of any kinematic gait curve, past multiplying the basis with the kinematic curves of a stride. Afterward, the Euclidean distance betwixt this kinematic gait bend and the average of a fix of healthy command strides were calculated, so that the deviation of a gait design from a normal gait contour was represented. Lastly, this value was scaled to improve the interpretability of the alphabetize, and so that every 10 points of GDI below 100 corresponded to 1 standard deviation (SD) abroad from the typical gait kinematics, whereas a score ≥ 100 represented a normal gait profile.
The GDI for our sample population was calculated for each step in both groups, subjects with iSCI and HV group, using the orthonormal basis provided in Schwartz and Rozumalski (2008). HV group data, used as the reference gait blueprint to compute the gait deviation, were collected post-obit the aforementioned process used with the individuals with iSCI. Each 3D kinematic gait analysis was associated to a SS WISCI II level according to the preferential condition to walk declared past the participants with iSCI. GDI information were grouped according to the respective WISCI Two level and HV group data were considered as an additional set. Normal distribution for each group was assessed with Kolmogorov-Smirnov tests. To facilitate the analysis, a histogram of the GDI data comprised inside each WISCI II level was calculated with a normal distribution curve fitted to its mean and SD. Afterward, one-way ANOVA tests were performed between the GDI values of each pair of WISCI II levels to identify differences among groups. P-value was set to p < 0.05 for all statistical procedures. All the data analysis was performed with Matlab R2019a (The MathWorks, Inc., Natick, MA, United States).
Results
Thirty-four (n = 34) adults with iSCI and fifty (n = l) HV met the inclusion criteria (Table i). Clinical characteristics of individuals with iSCI are shown in Table two. The dataset of iSCI sample included the post-obit WISCI II levels: 12, 13, 15, 16, 18, 19, and 20.

Tabular array i. Characteristics of individuals recruited for the study.
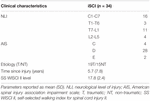
Table 2. Clinical characteristics of individuals with iSCI.
The analysis showed that GDI data were normally distributed beyond all WISCI II levels and also in the HV group. Tabular array 3 presents the number of strides, the range, the mean, and the SD of GDI values comprised in each WISCI II level. Results showed a trend of increasing average GDI values with decreasing level of walking damage in WISCI II levels 13 to 20 and the HV grouping, except in level xviii, whose average GDI was lower than the boilerplate on level 16. This can be easily seen in Figure ii, that shows the histograms of the GDI values stratified by WISCI II levels. Statistically significant differences were plant between HV group and all WISCI Ii levels. Nevertheless, they were only plant between WISCI II levels 13, 19, and xx. No statistically significant differences were establish between levels under 18 (inclusive), except by level 13. Therefore, the increasing human relationship between the GDI values and WISCI II levels was simply discriminative in the highest levels in subjects with iSCI (WISCI Two xix: lxx.2 ± 8.1; WISCI II 20: 80.4 ± xv.2), merely not in the lower levels, except in WISCI Two level 13 (47.1 ± 1.8).

Table 3. Descriptive statistics of GDI values (mean and range) within each WISCI Two level.
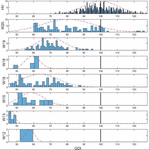
Effigy two. Histograms of GDI values stratified by WISCI II levels in population with iSCI. The dotted line represents the normal distribution curve fitted to the data wihtin each WISCI Two level. The vertical black line indicates the HV mean.
Give-and-take
Our results showed an increasing relationship betwixt the GDI values and WISCI 2 levels from 13 to 20, and the HV group, except for level 18. Nonetheless, results of the written report showed that the awarding of the GDI only distinguished WISCI II levels 13 (gait assisted with a walker), 19 (gait assisted with a cane), and 20 (no assistance required) from all the other WISCI II levels in developed population with iSCI. The alphabetize successfully distinguished all the individuals with iSCI from HV grouping. For those with WISCI Two level 20, GDI values were able to discriminate successfully an impaired gait, even if information technology did not required any external aid, from a normal gait blueprint. These results does not back up previous studies in which WISCI 2 showed a ceiling effect (Lemay and Nadeau, 2010; Wirz et al., 2010) and a better sensitivity to modify in spinal cord injured subjects with more impaired gait compared to those with higher levels of walking part (van Hedel et al., 2006). Regarding ranges of GDI values below WISCI II level xix, except for level 13, results showed an overlap betwixt the dissimilar levels, which indicates that the GDI compresses into a pocket-size range all WISCI 2 levels.
Birthday, our results bespeak that the GDI was not able to discriminate the functional diversity of adult population with iSCI related to walking harm defined past WISCI II levels. Therefore, results do non support our hypothesis, built upon the previous correlation analysis of the GDI with other clinical scales used in CP (Schwartz and Rozumalski, 2008; Molloy et al., 2010; Maanum et al., 2012; Massaad et al., 2014). Although more dumb gait patterns, lower GDI values, are associated with lower WISCI II levels, equally shown past the stratification, the differences between all levels are non statistically significant. Thus, the GDI is non a valid metric to distinguish the dissimilar walking harm levels divers by WISCI II in adult population with iSCI. These results may exist explained by several reasons. Beginning, because GDI gait features were originally obtained from 3D kinematic data of children with CP (Schwartz and Rozumalski, 2008), GDI could not be an appropriate index to study gait functionality in developed population with SCI. Therefore, application of the GDI in other pathologies dissimilar from CP should be washed with circumspection. Second, WISCI II considers gait impairment in terms of physical assistance, AADs, and orthoses required to walk ten grand, but without providing information apropos articulation kinematics related to limb coordination. Thus, other walking ability effect measures different from WISCI II are necessary to embrace the whole functional spectrum of walking ability in population with iSCI, such as categorical and spatiotemporal-related walking and balance measures (Sinovas-Alonso et al., 2021).
This written report brings to lite the existing lack of scientific literature in relation to an overall gait alphabetize that covers the functional diversity of patients with iSCI. This may be due to the fact that gait patterns in iSCI are very heterogeneous and variable depending on the level and severity of the lesion, making it difficult to establish a articulate pattern for the gear up of functional alterations that a field of study with iSCI may present. Knowledge of the most commonly contradistinct kinematic variables in iSCI would allow the creation of an overall gait index that could encompass the variety of functional alterations involved in iSCI patients' gait. This multivariate walking metric would permit a more accurate assessment of the development of patients with iSCI past quantifying the changes and, thus, assessing the quality of the therapeutic interventions carried out. Therefore, time to come work should aim at defining an overall gait index derived from 3D kinematic gait variables appropriate and specific for population with iSCI, focusing on its validation with other walking ability consequence measures.
There are several limitations in our work. The main i is related to the sample size in group with iSCI, which is reduced in some WISCI 2 levels and not homogeneous between the different levels and neither between iSCI and HV group. This reduced sample is related to the fact that funding lasted for one year of data gathering and we were not able to continue experimentation after July 2021. Sample size was as well reduced due to the wellness situation associated with the COVID-19 pandemic. Furthermore, this enquiry has considered gait maturation at the historic period of 16 years to ensure that young individuals with iSCI had accomplish a stable kinematics (Bleyenheuft and Detrembleur, 2012), which restricts the sample size of adults with iSCI included in the study. Another limitation of this report is related to the fact that during 3D kinematic gait analysis individuals with iSCI walked with the minimum external assistance required to walk safely. It means that some of the patients who usually wore orthoses or used canes to walk more comfortably did not use them since the context of the mensurate was to analyze gait with the least external interferences under medical prescription. Information technology is highly likely that GDI values accept been affected past this fact and, consequently, the relationship with the SS WISCI Two levels, which were sometimes different from those at the moment of the exam. Finally, due to the retrospective design of the report there is a lack of registration of other walking ability event measures, what has limited the report to the analysis of the relationship between the GDI and the WISCI 2.
The findings of this study indicated that the GDI is not an appropriate multivariate walking metric to represent the deviation of gait pattern in adult population with iSCI from a normal gait profile when it is compared with the levels of walking impairment described by the WISCI II. It is necessary to conduct further research into the evolution of a new overall gait alphabetize derived from SCI-specific 3D kinematic gait variables, involving a larger population, and validating it confronting other walking ability outcome measures such as categorical and spatiotemporal-related walking and rest measures.
Data Availability Statement
The raw data supporting the conclusions of this article will exist made available by the authors, without undue reservation.
Ideals Statement
The studies involving human participants were reviewed and approved by Local Ethics Commission of University Hospital Complex of Toledo, Espana. Written informed consent to participate in this study was provided past the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(southward) for the publication of whatever potentially identifiable images or data included in this article.
Author Contributions
DH-5, ÁG-A, and IS-A conceived the written report. IS-A and AR-One thousand registered data. DH-V analyzed data. IS-A and DH-V wrote the manuscript. RC-d-l-C and AJd-A contributed to the estimation and discussion of study results. AJd-A obtained funding. All authors revised and approved the final version of the manuscript, read and agreed to the published version of the manuscript.
Funding
This inquiry was supported by the TAILOR project: "Modular robotic and neuroprosthetic customizable systems for the assistance of pathological gait," funded by MICIN/AEI/10.13039/501100011033 and by "ERDF A manner of making Europe" (Grant No. RTI2018-097290-B-C31).
Disharmonize of Interest
The authors declare that the research was conducted in the absenteeism of any commercial or financial relationships that could be construed as a potential disharmonize of involvement.
Publisher's Note
All claims expressed in this article are solely those of the authors and practise not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this commodity, or merits that may be made past its manufacturer, is non guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge all participants (infirmary staff, students and patients) who accepted voluntarily to be part of this report.
References
Bani, M. A., Arazpour, M., Ghomshe, F. T., Mousavi, M. East., and Hutchins, S. W. (2013). Gait evaluation of the advanced reciprocating gait orthosis with solid versus dorsi flexion help ankle foot orthoses in paraplegic patients. Prosthet. Orthot. Int. 37, 161–167. doi: 10.1177/0309364612457704
PubMed Abstruse | CrossRef Full Text | Google Scholar
Bazarnik-Mucha, K., Snela, Southward.Ł, Szczepanik, M., Jarmuziewicz, A., Guzik, A., WoliŃska, O., et al. (2020). Three-dimensional analysis of gait in children and adolescents with juvenile idiopathic arthritis. Acta Bioeng. Biomech. 22, 35–45.
Google Scholar
Bleyenheuft, C., and Detrembleur, C. (2012). Kinematic covariation in pediatric, adult and elderly subjects: is gait control influenced by age? Clin. Biomech. 27, 568–572. doi: 10.1016/j.clinbiomech.2012.01.010
PubMed Abstract | CrossRef Full Text | Google Scholar
Broström, E. W., Esbjörnsson, A.-C., von Heideken, J., Larsson, P., Wretenberg, P., and Iversen, M. (2013). Change in Gait Departure Index after anti-tumour necrosis factor-α handling in individuals with rheumatoid arthritis: a pilot study. Scand. J. Rheumatol. 42, 356–361. doi: 10.3109/03009742.2013.776102
PubMed Abstract | CrossRef Full Text | Google Scholar
Burns, A. S., Delparte, J. J., Patrick, M., Marino, R. J., and Ditunno, J. F. (2011). The reproducibility and convergent validity of the walking index for spinal cord injury (WISCI) in chronic spinal string injury. Neurorehabil. Neural Repair 25, 149–157. doi: 10.1177/1545968310376756
PubMed Abstract | CrossRef Full Text | Google Scholar
Cimolin, V., Galli, M., Vimercati, S. L., and Albertini, G. (2011). Employ of the Gait Deviation Index for the assessment of gastrocnemius fascia lengthening in children with Cerebral Palsy. Res. Dev. Disabil. 32, 377–381. doi: 10.1016/j.ridd.2010.x.017
PubMed Abstruse | CrossRef Full Text | Google Scholar
Correa, M. P., Devetak, G. F., Martello, S. K., de Almeida, J. C., Pauleto, A. C., and Manffra, E. F. (2017). Reliability and Minimum Detectable Modify of the Gait Deviation Index (GDI) in mail-stroke patients. Gait Posture 53, 29–34. doi: x.1016/j.gaitpost.2016.12.012
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cupp, T., Oeffinger, D., Tylkowski, C., and Augsburger, S. (1999). Age-related kinetic changes in normal pediatrics. J. Pediatr. Orthop. 19, 475–478. doi: 10.1097/00004694-199907000-00010
PubMed Abstruse | CrossRef Total Text | Google Scholar
Ditunno, J. F., Barbeau, H., Dobkin, B. H., Elashoff, R., Harkema, S., Marino, R. J., et al. (2007). Validity of the walking scale for spinal cord injury and other domains of office in a multicenter clinical trial. Neurorehabil. Neural Repair 21, 539–550. doi: x.1177/1545968307301880
PubMed Abstract | CrossRef Full Text | Google Scholar
Ditunno, P. Fifty., Patrick, M., Stineman, Thousand., and Ditunno, J. F. (2008). Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord 46, 500–506. doi: 10.1038/sj.sc.3102172
PubMed Abstract | CrossRef Total Text | Google Scholar
Eriksson, Thou., Bartonek, Å, Pontén, East., and Gutierrez-Farewik, E. M. (2015). Gait dynamics in the wide spectrum of children with arthrogryposis: a descriptive study. BMC Musculoskelet. Disord. 16:384. doi: x.1186/s12891-015-0834-5
PubMed Abstruse | CrossRef Full Text | Google Scholar
Esbjörnsson, A.-C., Rozumalski, A., Iversen, M. D., Schwartz, Yard. H., Wretenberg, P., and Broström, Eastward. W. (2014). Quantifying gait deviations in individuals with rheumatoid arthritis using the Gait Deviation Index. Scand. J. Rheumatol. 43, 124–131. doi: x.3109/03009742.2013.822095
PubMed Abstract | CrossRef Full Text | Google Scholar
Eshraghi, A., Osman, N. A. A., Karimi, M., Gholizadeh, H., Soodmand, Eastward., and Abas, Westward. A. B. Due west. (2014). Gait Biomechanics of Individuals with Transtibial Amputation: issue of Intermission System. PLoS One 9:e96988. doi: x.1371/journal.pone.0096988
PubMed Abstruse | CrossRef Full Text | Google Scholar
Galli, K., Cimolin, V., De Pandis, M. F., Schwartz, M. H., and Albertini, One thousand. (2012). Apply of the Gait Deviation index for the evaluation of patients with Parkinson'southward affliction. J. Mot. Behav. 44, 161–167. doi: 10.1080/00222895.2012.664180
PubMed Abstract | CrossRef Full Text | Google Scholar
Ganley, K. J., and Powers, C. G. (2005). Gait kinematics and kinetics of 7-year-sometime children: a comparison to adults using age-specific anthropometric data. Gait Posture 21, 141–145. doi: 10.1016/j.gaitpost.2004.01.007
PubMed Abstract | CrossRef Full Text | Google Scholar
Garman, C. R., Graf, A., Krzak, J., Caudill, A., Smith, P., and Harris, Chiliad. (2019). Gait Deviations in Children With Osteogenesis Imperfecta Blazon I. J. Pediatr. Orthop. 39, e641–e646. doi: x.1097/BPO.0000000000001062
PubMed Abstract | CrossRef Full Text | Google Scholar
Guzik, A., and Drużbicki, M. (2020). Application of the Gait Deviation Index in the analysis of postal service-stroke hemiparetic gait. J. Biomech. 99:109575. doi: 10.1016/j.jbiomech.2019.109575
PubMed Abstruse | CrossRef Full Text | Google Scholar
Hwang, M., Flanagan, A., Graf, A., Kruger, Thou. G., Scullion, N., Tayne, Due south., et al. (2021). Gait Characteristics in Youth With Transverse Myelitis. Meridian Spinal Cord Inj. Rehabil. 27, 38–48. doi: 10.46292/sci20-00048
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ito, T., Noritake, Grand., Sugiura, H., Kamiya, Y., Tomita, H., Ito, Y., et al. (2019). Association between Gait Deviation Index and Physical Function in Children with Bilateral Spastic Cerebral Palsy: a Cantankerous-Sectional Study. J. Clin. Med. 9:E28. doi: 10.3390/jcm9010028
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ito, T., Sugiura, H., Ito, Y., Noritake, K., and Ochi, Northward. (2021). Effect of the COVID-19 Emergency on Physical Function amidst School-Aged Children. Int. J. Environ. Res. Public Health xviii:9620. doi: 10.3390/ijerph18189620
PubMed Abstract | CrossRef Full Text | Google Scholar
Ito, Y., Ito, T., Kurahashi, Northward., Ochi, N., Noritake, K., Sugiura, H., et al. (2020). Gait characteristics of children with Williams syndrome with dumb visuospatial recognition: a 3-dimensional gait assay study. Exp. Brain Res. 238, 2887–2895. doi: x.1007/s00221-020-05946-0
PubMed Abstract | CrossRef Full Text | Google Scholar
Jackson, A. B., Carnel, C. T., Ditunno, J. F., Read, K. S., Boninger, Chiliad. L., Schmeler, M. R., et al. (2008). Outcome measures for gait and ambulation in the spinal cord injury population. J. Spinal Cord Med. 31, 487–499. doi: 10.1080/10790268.2008.11753644
PubMed Abstract | CrossRef Full Text | Google Scholar
Kark, L., Odell, R., McIntosh, A. S., and Simmons, A. (2016). Quantifying prosthetic gait departure using simple outcome measures. World J. Orthop. seven, 383–391. doi: 10.5312/wjo.v7.i6.383
PubMed Abstract | CrossRef Full Text | Google Scholar
Kirshblum, S. C., Burns, S. P., Biering-Sorensen, F., Donovan, W., Graves, D. E., Jha, A., et al. (2011). International standards for neurological classification of spinal string injury (Revised 2011). J. Spinal Cord Med. 34, 535–546. doi: 10.1179/204577211X13207446293695
PubMed Abstract | CrossRef Total Text | Google Scholar
Kobsar, D., Charlton, J. Yard., and Hunt, M. A. (2019). Individuals with knee osteoarthritis present increased gait pattern deviations as measured by a articulatio genus-specific gait deviation alphabetize. Gait Posture 72, 82–88. doi: 10.1016/j.gaitpost.2019.05.020
PubMed Abstract | CrossRef Total Text | Google Scholar
Lemay, J.-F., and Nadeau, S. (2010). Standing balance assessment in ASIA D paraplegic and tetraplegic participants: concurrent validity of the Berg Balance Scale. Spinal String 48, 245–250. doi: 10.1038/sc.2009.119
PubMed Abstract | CrossRef Total Text | Google Scholar
Maanum, 1000., Jahnsen, R., Stanghelle, J. G., Sandvik, L., Larsen, K. L., and Keller, A. (2012). Face up and construct validity of the Gait Deviation Index in adults with spastic cerebral palsy. J. Rehabil. Med. 44, 272–275. doi: 10.2340/16501977-0930
PubMed Abstract | CrossRef Total Text | Google Scholar
Malt, Thou. A., Aarli, Å, Bogen, B., and Fevang, J. M. (2016). Correlation betwixt the Gait Deviation Index and gross motor function (GMFCS level) in children with cerebral palsy. J. Child Orthop. 10, 261–266. doi: 10.1007/s11832-016-0738-4
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mar, D., Lieberman, I., and Haddas, R. (2019). The Gait Difference Index as an indicator of gait abnormality among degenerative spinal pathologies. Eur. Spine J. 29, 2591–2599. doi: 10.1007/s00586-019-06252-ii
PubMed Abstruse | CrossRef Full Text | Google Scholar
Marino, R. J., Scivoletto, Thou., Patrick, M., Tamburella, F., Read, G. Due south., Burns, A. S., et al. (2010). Walking index for spinal cord injury version ii (WISCI-Two) with repeatability of the 10-chiliad walk fourth dimension: inter- and intrarater reliabilities. Am. J. Phys. Med. Rehabil. 89, 7–xv. doi: 10.1097/PHM.0b013e3181c560eb
PubMed Abstract | CrossRef Full Text | Google Scholar
Massaad, A., Assi, A., Skalli, W., and Ghanem, I. (2014). Repeatability and validation of Gait Divergence Index in children: typically developing and cerebral palsy. Gait Posture 39, 354–358. doi: ten.1016/j.gaitpost.2013.08.001
PubMed Abstract | CrossRef Full Text | Google Scholar
McGinley, J. L., Bakery, R., Wolfe, R., and Morris, K. E. (2009). The reliability of three-dimensional kinematic gait measurements: a systematic review. Gait Posture 29, 360–369. doi: 10.1016/j.gaitpost.2008.09.003
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mindler, K. T., Kranzl, A., Stauffer, A., Haeusler, M., Ganger, R., and Raimann, A. (2020). Affliction-specific gait deviations in pediatric patients with 10-linked hypophosphatemia. Gait Posture 81, 78–84. doi: 10.1016/j.gaitpost.2020.07.007
PubMed Abstruse | CrossRef Full Text | Google Scholar
Molloy, M., McDowell, B. C., Kerr, C., and Cosgrove, A. P. (2010). Further evidence of validity of the Gait Divergence Index. Gait Posture 31, 479–482. doi: ten.1016/j.gaitpost.2010.01.025
PubMed Abstruse | CrossRef Full Text | Google Scholar
Morganti, B., Scivoletto, G., Ditunno, P., Ditunno, J. F., and Molinari, Grand. (2005). Walking index for spinal cord injury (WISCI): criterion validation. Spinal String 43, 27–33. doi: 10.1038/sj.sc.3101658
PubMed Abstruse | CrossRef Total Text | Google Scholar
Murphy, A. T., Kravtsov, Southward., Sangeux, M., Rawicki, B., and New, P. W. (2019). Utilizing three dimensional clinical gait analysis to optimize mobility outcomes in incomplete spinal cord damage. Gait Posture 74, 53–59. doi: x.1016/j.gaitpost.2019.08.001
PubMed Abstruse | CrossRef Full Text | Google Scholar
Quadri, S. A., Farooqui, 1000., Ikram, A., Zafar, A., Khan, 1000. A., Suriya, S. S., et al. (2020). Recent update on basic mechanisms of spinal string injury. Neurosurg. Rev. 43, 425–441. doi: 10.1007/s10143-018-1008-three
PubMed Abstruse | CrossRef Total Text | Google Scholar
Rasmussen, H. Thousand., Pedersen, N. W., Overgaard, S., Hansen, L. K., Dunkhase-Heinl, U., Petkov, Y., et al. (2019). Gait analysis for individually tailored interdisciplinary interventions in children with cerebral palsy: a randomized controlled trial. Dev. Med. Child Neurol. 61, 1189–1195. doi: ten.1111/dmcn.14178
PubMed Abstract | CrossRef Full Text | Google Scholar
Rosenlund, South., Holsgaard-Larsen, A., Overgaard, S., and Jensen, C. (2016). The Gait Deviation Index Is Associated with Hip Muscle Strength and Patient-Reported Outcome in Patients with Severe Hip Osteoarthritis-A Cross-Exclusive Study. PLoS One 11:e0153177. doi: ten.1371/journal.pone.0153177
PubMed Abstract | CrossRef Full Text | Google Scholar
Sagawa, Y., Watelain, E., De Coulon, G., Kaelin, A., Gorce, P., and Armand, S. (2013). Are clinical measurements linked to the Gait Deviation Index in cerebral palsy patients? Gait Posture 38, 276–280. doi: 10.1016/j.gaitpost.2012.11.026
PubMed Abstract | CrossRef Total Text | Google Scholar
Scivoletto, G., Miscusi, K., Forcato, South., Ricciardi, 50., Serrao, G., Bellitti, R., et al. (2017). The Rehabilitation of Spinal Cord Injury Patients in Europe. Acta Neurochir. Suppl. 124, 203–210. doi: x.1007/978-3-319-39546-3_31
CrossRef Full Text | Google Scholar
Scivoletto, G., Tamburella, F., Laurenza, L., Foti, C., Ditunno, J. F., and Molinari, M. (2011). Validity and reliability of the 10-m walk examination and the 6-min walk exam in spinal string injury patients. Spinal Cord 49, 736–740. doi: 10.1038/sc.2010.180
PubMed Abstract | CrossRef Total Text | Google Scholar
Scivoletto, K., Tamburella, F., Laurenza, L., Torre, Chiliad., Molinari, M., and Ditunno, J. F. (2014). Walking Index for Spinal String Injury version II in astute spinal cord injury: reliability and reproducibility. Spinal Cord 52, 65–69. doi: x.1038/sc.2013.127
PubMed Abstruse | CrossRef Total Text | Google Scholar
Sienko Thomas, S., Buckon, C. E., Nicorici, A., Bagley, A., McDonald, C. M., and Sussman, Chiliad. D. (2010). Classification of the gait patterns of boys with Duchenne muscular dystrophy and their human relationship to function. J. Child Neurol. 25, 1103–1109. doi: ten.1177/0883073810371002
PubMed Abstract | CrossRef Full Text | Google Scholar
Sinovas-Alonso, I., Gil-Agudo, Á, Cano-de-la-Cuerda, R., and del-Ama, A. J. (2021). Walking Ability Consequence Measures in Individuals with Spinal Cord Injury: a Systematic Review. Int. J. Environ. Res. Public Health xviii:9517. doi: 10.3390/ijerph18189517
PubMed Abstract | CrossRef Full Text | Google Scholar
Speciali, D. S., de Oliveira, E. Yard., dos Santos, N. K., Pereira, F. 5., Fracini, A. C., Fukuda, T. Y., et al. (2013). Use of the Gait Deviation Index and spatiotemporal variables for the assessment of dual job interference prototype. J. Bodyw. Mov. Ther. 17, 19–27. doi: 10.1016/j.jbmt.2012.03.001
PubMed Abstruse | CrossRef Full Text | Google Scholar
Trivedi, J., Srinivas, S., Trivedi, R., Davidson, Due north., Munigangaiah, Due south., Bruce, C., et al. (2021). Preoperative and Postoperative, Three-dimensional Gait Assay in Surgically Treated Patients With High-grade Spondylolisthesis. J. Pediatr. Orthop. 41, 111–118. doi: ten.1097/BPO.0000000000001721
PubMed Abstract | CrossRef Full Text | Google Scholar
van Hedel, H. J. A., Wirz, Thou., and Curt, A. (2006). Improving walking cess in subjects with an incomplete spinal cord injury: responsiveness. Spinal Cord 44, 352–356. doi: 10.1038/sj.sc.3101853
PubMed Abstract | CrossRef Full Text | Google Scholar
Wilson, Northward. C., Signal, Due north., Naude, Y., Taylor, D., and Stott, North. South. (2015). Gait Deviation Index Correlates With Daily Stride Activity in Children With Cerebral Palsy. Arch. Phys. Med. Rehabil. 96, 1924–1927. doi: ten.1016/j.apmr.2015.05.024
PubMed Abstract | CrossRef Total Text | Google Scholar
Wirz, One thousand., Müller, R., and Bastiaenen, C. (2010). Falls in persons with spinal cord injury: validity and reliability of the Berg Rest Scale. Neurorehabil. Neural Repair 24, 70–77. doi: ten.1177/1545968309341059
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhou, C., Xia, H., Yin, J., and Zheng, Y. (2021). Three-dimensional gait quantitative analysis in postoperative rehabilitation of lumbar degenerative diseases: a self-controlled before-subsequently written report. Am. J. Transl. Res. thirteen, 6913–6920.
Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fnhum.2022.826333/full
0 Response to "Cannot Access Article to Review on Gait & Posture"
Post a Comment